Abstract
Introduction
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia (AIHA). Although there is consensus on an increased risk of thromboembolic events (TEs) in patients with CAD, the evidence for the effect on mortality is mixed. The objectives of this retrospective study were two-fold: 1) to evaluate whether patients with CAD in the US have a higher risk of mortality and TEs compared with a matched non-CAD population, and 2) to determine the association between CAD biomarkers and the risk of mortality and TEs.
Methods
This was a retrospective, cohort study of patients with CAD and propensity score-matched patients without CAD in the US, identified using the OPTUM Electronic Health Record database (Jan 2007-Jan 2021). The CAD cohort included patients who had ≥1 medical encounter with an AIHA-related diagnosis code, ≥3 separate mentions of CAD, ≥1 non-negative sentiment associated with CAD, and ≥1 healthcare visit prior to index date (first CAD mention). Patients with cold agglutinin syndrome were excluded. Mortality and TEs were assessed over the follow-up period (from index date to end of medical activity, study period, or death). Biomarker levels were assessed at baseline and during follow-up, and categorized as follows: elevated bilirubin, >1.2 mg/dL; elevated lactate dehydrogenase (LDH), >250 U/L; and hemoglobin (Hb), mild anemia 10≤ Hb <12 g/dL, moderate 8 ≤Hb <10 g/dL, and severe <8 g/dL. For objective 1, adjusted incidence rate ratios (aIRRs) for mortality and TEs were obtained using multivariate Poisson regressions. Probability of survival and remaining TE-free were assessed using Kaplan-Meier analysis with log-rank testing; adjusted hazard ratios (aHRs) and 95% CIs were obtained using multivariate Cox proportional hazards regression analysis. For objective 2, time-varying Cox regressions were used in the CAD cohort to analyze the relationship between CAD biomarkers (all observations until event) and risk of mortality or TE. Confounding variables, such as smoking, comorbidities, past TEs, and index season, were accounted for in the analyses (adjusted ratios).
Results
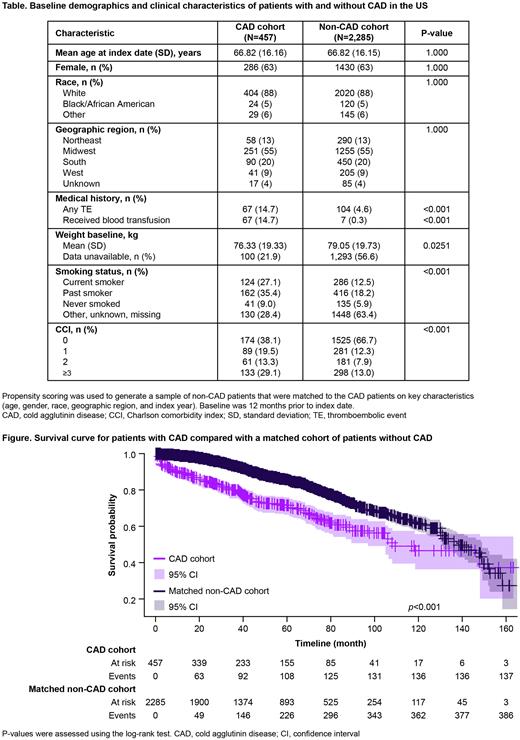
Overall, 457 patients with CAD and 2285 matched patients without CAD were included. Median follow-up was 40.23 (0.16; 163.39) and 48.16 (0.07; 164.90) months, respectively. During follow-up, 30.0% of patients with CAD died compared with 16.9% in the non-CAD cohort. At baseline, 14.7% of patients with CAD and 4.6% without CAD had a history of TE (Table). During follow-up, 33.5% of patients with CAD compared with 18.0% in the non-CAD cohort experienced a TE. aIRRs [95% CI] were 2.19 [1.78; 2.67] for mortality and 2.24 [1.83; 2.72] for TEs. Overall, survival probability was significantly lower in the CAD cohort than in the non-CAD cohort during full follow-up (Figure), and at 12-, 24-, and 36-months (all p<0.001). Mortality in the CAD cohort was twice that of the non-CAD cohort (aHR: 2.21 [1.81; 2.71]; p<0.001). Similarly, probability to remain TE-free was significantly lower for patients with CAD than those without during full follow-up and at 12-, 24-, and 36-months (all p<0.001). Risk of TEs in the CAD cohort was twice that of the non-CAD cohort (aHR: 2.13 [1.75; 2.59]; p<0.001).
In the CAD cohort, patients with mild, moderate, or severe anemia had a significantly higher risk of mortality than those without anemia (aHR: 2.71 [1.48; 4.95]; 7.23 [4.12; 12.69]; and 17.78 [9.72; 32.51], respectively; all p<0.001). Moderate and severe anemia were also associated with increased risk of TEs compared with no anemia (aHR: 2.19 [1.28; 3.72] and 3.84 [2.01; 7.35], respectively; all p<0.001); mild anemia did not reach significance (1.37 [0.82; 2.29]; p=0.235). Elevated bilirubin levels significantly increased the risk of mortality compared with normal levels (aHR: 1.59 [1.10; 2.30]; p=0.013). Elevated LDH somewhat increased the risk of mortality compared with normal levels (aHR: 1.44 [0.96; 2.17]; p=0.076).
Conclusion
The clinical burden associated with CAD is significant and extends beyond anemia. Patients with CAD were two times more likely to die or experience TEs than those without CAD. Within the CAD cohort, the degree of ongoing hemolysis, as measured by increased bilirubin, and subsequent anemia contributed to an increased mortality risk. Moderate and severe anemia also increased the risk of a TE. Chronic control of complement activation and the resulting hemolysis in CAD may therefore help manage the risk of mortality and TEs.
Disclosures
Broome:Dianthus: Consultancy; Alexion: Honoraria, Research Funding; Argenx: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Annexon: Research Funding; Novartis: Research Funding; Incyte: Honoraria, Research Funding; Electra: Research Funding. Barcellini:Biocryst: Honoraria; Agios: Honoraria, Research Funding; Alexion: Honoraria; Apellis: Honoraria; SOBI: Honoraria; Novartis: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Momenta: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Speakers Bureau. Roeth:Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Biocryst: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding; Apellis Pharmaceuticals: Consultancy, Honoraria. Karaouni:BMAPS SARL (Geneva, Switzerland): Current Employment; Sanofi: Consultancy. Joly:Sanofi: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Yoo:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Terlinden:Sanofi: Current Employment, Current equity holder in publicly-traded company. Adeyemi:Sanofi: Ended employment in the past 24 months. Moride:Sanofi: Consultancy; Yolarx Consultants: Current Employment. Hill:Alexion: Honoraria; ReAlta: Honoraria; Incyte: Honoraria; Immunovant: Honoraria; Janssen: Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Argenx: Consultancy; Apellis: Consultancy, Honoraria; Amgen: Honoraria; Grifols: Consultancy, Honoraria; Sobi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal